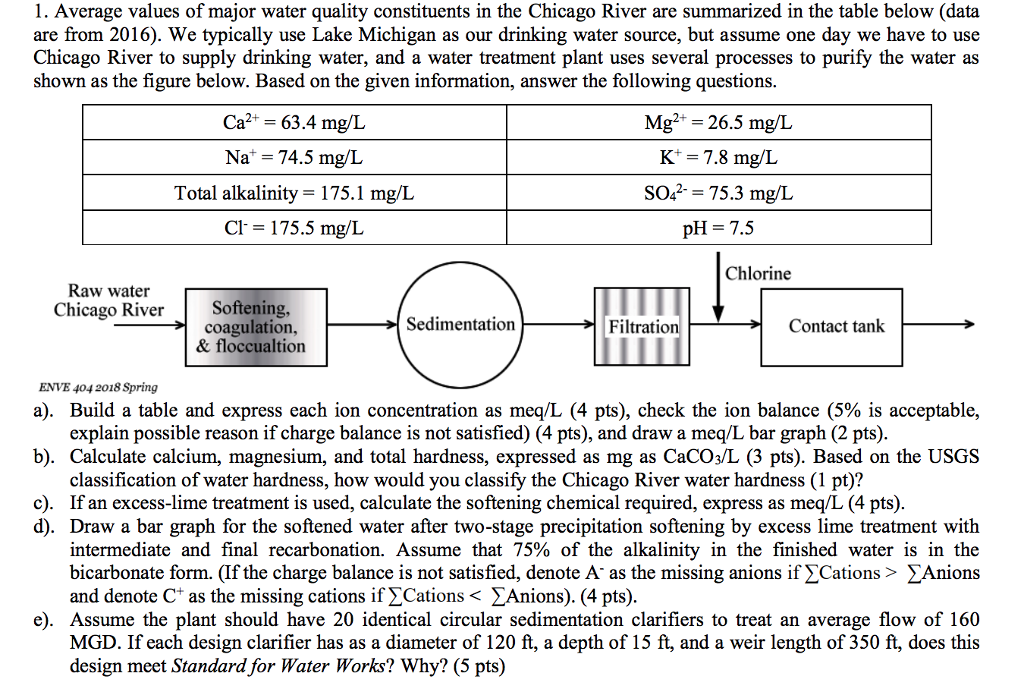
Chicago Water Hardness Mg L

Symptoms of hard water include.
Chicago water hardness mg l. Mg l means milligrams per liter note. What is soft water. A grain of water hardness is comparable to 1 7000th of a pound. Hard water has a noticeably unpleasant flavor but is still potable.
If a test for hard water is measured in parts per million or milligrams per liter you can take the total hardness level and divide it by 17 1 to get hardness in grains per gallon. For example if your water test shows 250 mg l hardness you actually have 14 62 grains per gallon. This water hardness scale build up comes at a significant cost. The hardness of groundwater supplies was higher and levels ranged from 40 to 1300 mg l with an average of 294 mg l.
Hard water usually requires water softening equipment to make it usable for a home. Hardness is usually expressed in grains per gallon or ppm as calcium carbonate equivalent. This map uses ppm measures. General guidelines for classification of waters are.
The molar mass of caco 3 ca 2 and mg 2 are respectively 100 1 g mol 40. Total permanent water hardness is calculated with the following formula. The calcium and magnesium hardness is the concentration of calcium and magnesium ions expressed as equivalent of calcium carbonate. Grains per gallon is the business standard approach for talking about water hardness.
Hard water causes scale to accumulate in pipe systems and equipment. In ontario the hardness of drinking water from surface sources ranged from 3 7 to 296 mg l with an average of 95 mg l. Total permanent hardness calcium hardness magnesium hardness. Ppm and mg l are the same thing.
Water that is classified as hard has 150 300 ppm of minerals dissolved in it. 61 to 120 mg l as moderately hard. Soft water is defined by american national standards nsf ansi 44 and nsf ansi 330 as water containing 1 grain of hardness per gallon or 17 1 mg l hardness. Contingent upon where a water test is performed this data will be accounted for in mg l milligrams per liter or gpg grains per gallon.
Cleaning agents do not react well with hard water and often a distinct oily white crust is left behind after cleaning. Treated or softened water 0 1 gpg total grains x 17 1 equals ppm slightly hard 1 2 gpg total grains x 17 1 equals ppm medium hard 3 7 gpg total grains x 17 1 equals ppm hard 7 10 5 gpg total grains x 17 1 equals ppm. And more than 180 mg l as very hard. 0 to 60 mg l milligrams per liter as calcium carbonate is classified as soft.
Jumping mg l by 17 1 will change it to gpg. 121 to 180 mg l as hard. Hardness is caused by compounds of calcium and magnesium and by a variety of other metals.